Technological and socioeconomic progress has impacted healthcare systems and its antecedents since always. Yet, those dynamics are increasingly volatile and fast paced. For example, population is ageing rapidly in the developed - and to some extent in the developing - world. On top of that, black swan events like the Covid pandemic and the Russian incursion in Ukraine, put additional strain on national healthcare systems (and the underlying economies). While healthcare decision makers are painfully aware of the need for transformation, they are often restricted by political and budgetary boundaries.
Healthcare systems under pressure to modernize its ageing structures
In the post-World-War-II-world healthcare was centred around one-time interventions or (few) periodic treatments. Contagious diseases and work accidents seemed to be the priority. Since then, the need for continuous management of civilization diseases like e.g. heart failure or diabetes as well as disease prevention is growing. Nonetheless, healthcare systems are in large still set up like they were in the post-world-war-II world. Although, significant progress on drug and therapy development level happened, healthcare service delivery is lagging and patient centricity remains an abstract concept in most cases. So how can modern day healthcare systems increase resilience, ensure sustainability and most importantly increase patient quality of life?
While intrinsic improvement and clever reforms are a viable path, they are often unambitious and slow. Private innovation stemming from both long-established corporates and upcoming start-ups can be a powerful complement. EIT Health – an EU backed healthcare innovation network – recommends enabling collaborative environments for this very reason. Such environments should involve various societal stakeholders including patients, policymakers, and industry. The pharma industry has been innovation heavy weights for a while now – both in terms of filed patents and implemented product and process improvements. While established pharma companies churn out new molecules and novel therapies, they are lacking the experience to innovate “beyond the pill”. Medical technology firms are increasingly emerging and complementing pharma know-how with more engineering and software-oriented expertise. This results in a surge of novel medical devices, digital therapeutics, software and medical information systems.
New “outside” ideas can improve treatment delivery while achieving cost savings
Regarding MedTech, we see a surge of cost-effective aid-to-diagnosis and diagnostic devices. Such products can greatly help early diagnosis and kick off treatment at an early stage and help avoiding costly hospitalizations and acute interventions. In regions with underdeveloped medical infrastructure, lower cost alternatives to cost-prohibitive devices can enable healthcare practitioners (HCPs) to perform assessments, which were previously associated with long waiting times or regional exclusivity. On the individual level, software solutions like AI/AR apps can help HCPs to interpreting patient data and spot rare diseases.
Spotlight Powerful Medical
 Powerful Medical - a Slovakia based start-up - and their flagship product PMCardio enable HCPs to interpret standard ECG charts more reliably. The healthcare practitioners simply “scans” the print-out with the help of their smartphone and within seconds the PMCardio algorithm digitizes the data, suggests next steps to finalized diagnosis or initiate treatment according to the latest cardiology guidelines.
Powerful Medical - a Slovakia based start-up - and their flagship product PMCardio enable HCPs to interpret standard ECG charts more reliably. The healthcare practitioners simply “scans” the print-out with the help of their smartphone and within seconds the PMCardio algorithm digitizes the data, suggests next steps to finalized diagnosis or initiate treatment according to the latest cardiology guidelines.
On a systematic level, integrated medical information systems can help healthcare practitioners in individual or group practice setting as well as in medical institutions like hospitals. The European Parliament has assessed several key areas, where medical information systems and AI can benefit greatly. In addition to clinical practice and biomedical research, software solutions can support health administration and patient flow management. Effective digitization and clean-up of existing data as well as secure communication channels between HCPs across medical institutions and will facilitate collaboration.
Spotlight Philips ICU telemedicine program

As an example, Philips has established remote monitoring systems in US hospitals, which allowed for more effective oversight and support during night and weekend shifts. This resulted in savings of $4.6m over the course of 15 months and an increase in discharges to home healthcare. At the same time discharge to long-term care hospitals and patient re-admissions decreased.
Moreover, digital tools can be combined with conventional medication, allowing for more effective and better personalized treatment and monitoring both in traditional and telehealth setting.
Committed collaboration among public and private players is key for healthcare modernization
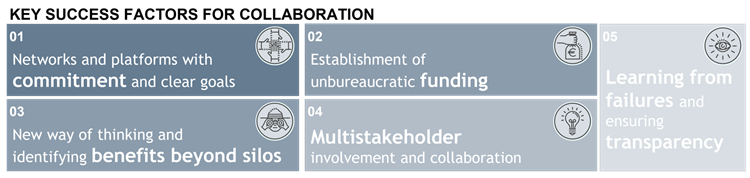
The innovation opportunities in the pharma and MedTech industry can improve patient quality of life and reduce costs on the healthcare system. Yet, in practice such innovation oriented public-private-partnerships often underperform. What success factors do we need to nurture private innovation and effectively implement in the healthcare systems? First, a collaborative environment must be established. While this sounds abstract, in practice networks and platforms including industry, regulators, HCPs and public bodies need to be established and equipped with sufficient resources and a clear goal in mind. Second, funding opportunities must be available with minimal bureaucratic constraints. In combination with local CVC, VC and angel investors, public grants and other form of governmental funding should be considered. Third, probably the most difficult aspect of enabling open innovation in healthcare systems, we need to break up cognitive and organizational silos. Decision makers must learn not to limit themselves to historical processes within their traditional field of expertise. Instead, they need to understand value propositions on system-wide level. Fourth, for effective open innovation, central stakeholders outside of R&D departments must be included in the development and implementation process. Central stakeholders include, but are not limited to patients, HCPs and clinic administrators who all provide a different perspective and valuable inputs. Finally, we must not forget culture change. Innovative ventures can and do fail often. We must learn to accept failure, be transparent about it and most importantly learn from it – on the product, process and system level.
Summary:
- Healthcare systems in many cases are still built on top of post-World-War-II structures and therefore lagging in value delivery and patient centricity despite monumental progress in reg. available therapeutic methods
- At the same time pharma and MedTech industry is producing innovation like almost no other industry on product and process level, including both hardware and software elements
- Public-private-partnerships are a viable method to harness industry innovation and implement it in HC system thereby increasing its performance and reducing costs
- Yet, such an open innovation approach requires commitment, effective governance, funding, and true collaboration – both during development and implementation
Outlook
It is clear that strategic collaborations as well as external innovations in the healthcare and pharmaceutical industries play a massive role in improving patients’ lives. To achieve successful partnerships, big pharma companies are required to recreate their current business models. You might wonder, how it could occur and what issues corporations should take into consideration. To reveal the details, you will find more information in our next article at 15. December!
Interested in these topics? Read more articles about Health Care here: #majorpharmadisruptions
Author:
Maxim Lebedev

maxim.lebedev@bdo.at
+43 5 70 375 - 1287